The Lewis structure of BrCl contains a single bond between the bromine atom and chlorine atom, and both the atoms have three lone pairs.
Alternative method: BrCl Lewis structure
Steps
Draw skeleton
In this step, first calculate the total number of valence electrons. And then, decide the central atom.
- Let’s calculate the total number of valence electrons
We know that… both bromine and chlorine are the group 17 elements. Hence, both bromine and chlorine have seven valence electrons.
Now BrCl has one bromine atom and one chlorine atom.
So the total number of valence electrons = valence electrons of bromine atom + valence electrons of chlorine atom
Therefore, the total number of valence electrons = 7 + 7 = 14
- Now decide the central atom
The atom with the least electronegative value is placed at the center. By looking at the periodic table, we get the electronegativity values for bromine and chlorine as follows:
Electronegativity value of bromine = 2.96
Electronegativity value of chlorine = 3.16
Obviously, bromine is less electronegative than chlorine. Hence, assume that bromine is the central atom.
So now, put bromine and chlorine next to each other. And draw the rough skeleton structure for the Lewis structure of BrCl something like this:
Also read: How to draw Lewis structure of BrCN (5 steps)
Show chemical bond
Place two electrons between the atoms to show a chemical bond as follows:
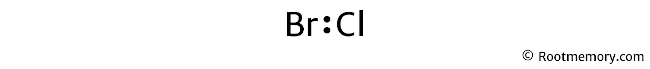
Also read: How to draw Lewis structure of AsCl3 (4 steps)
Mark lone pairs
As calculated earlier, we have a total of 14 valence electrons. And in the above structure, we have already used two valence electrons. Hence, twelve valence electrons are remaining.
Two valence electrons represent one lone pair. So twelve valence electrons = six lone pairs.
Note that bromine is a period 4 element, so it can keep more than 8 electrons in its last shell. And chlorine is a period 3 element, so it can keep more than 8 electrons in its last shell.
Also, make sure that you start marking these lone pairs on outside atoms first. And then, on the central atom.
The outside atom is chlorine, so chlorine will get three lone pairs. And the central atom (bromine) will also get three lone pairs.
So the Lewis structure of BrCl looks something like this:
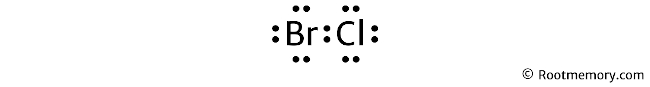
In the above structure, you can see that the octet is completed on the central atom (bromine), and also on the outside atom. Therefore, the octet rule is satisfied.
After completing the octet, one last thing we need to do is, calculate the formal charge and check the stability of the above structure.
Also read: How to draw Lewis structure of XeH4 (4 steps)
Calculate formal charge and check stability
The following formula is used to calculate the formal charges on atoms:
Formal charge = valence electrons – nonbonding electrons – ½ bonding electrons
Collect the data from the above structure and then, write it down below as follows:
- For bromine atom
Valence electrons = 7
Nonbonding electrons = 6
Bonding electrons = 2
Formal charge = 7 – 6 – ½ (2) = 0
- For chlorine atom
Valence electrons = 7
Nonbonding electrons = 6
Bonding electrons = 2
Formal charge = 7 – 6 – ½ (2) = 0
Mention the formal charges of atoms on the structure. So the Lewis structure of BrCl looks something like this:
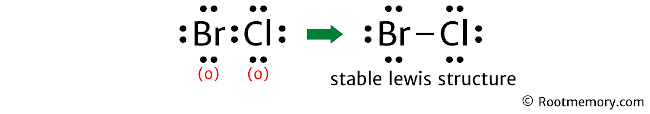
In the above structure, you can see that the formal charges of both (bromine and chlorine) are zero. Therefore, this is the stable Lewis structure of BrCl.
And the horizontal line drawn in the above structure represents a pair of bonding valence electrons.
Next: Lewis structure of BrCN
Related
- Lewis structure of BrCN
- Lewis structure of AsCl3
- Lewis structure of XeH4
- Lewis structure of BeI2
- Lewis structure of SiCl2Br2
External video
- How to Draw the Lewis Dot Structure for BrCl: Bromine monochloride – YouTube • Wayne Breslyn
External links
- BrCl Lewis Structure in 5 Steps (With Images) – Pediabay
- BrCl Lewis Structure, Geometry, Hybridization, and Polarity – Techiescientist
- Drawing the Lewis Structure for BrCl3 – The Geoexchange
- Draw the Lewis structure for BrCl – Chegg
- Determine whether BrCl would be best represented by an ionic or covalent Lewis structure – Homework.Study.com
- BrCl Lewis dot structure – Answers
- Draw the Lewis structure for BrCl molecule, predict the molecular shape and hybridization on the central atom and indicate whether the molecule is polar or nonpolar – Numerade
Deep
Rootmemory.com was founded by Deep Rana, who is a mechanical engineer by profession and a blogger by passion. He has a good conceptual knowledge on different educational topics and he provides the same on this website. He loves to learn something new everyday and believes that the best utilization of free time is developing a new skill.
